1 SUMMARY
Due to growing demand of sustainable fuels the demand for storage of alcohols has increased significantly in the recent years. Often larger quantities of these essential liquids are stored in aboveground storage tanks with internal floating roofs (IFR). These roofs are used to reduce emissions to the air and prevent explosive atmospheres inside the tanks. Quite often there are significant discussions about suitability of aluminium structures for partially submerged service in Methanol or Ethanol.
In this respect a number of aspects are important:
- Construction of the roof
- Construction materials
- Water content and quality of the stored product.
Suitability for service is therefore dependent on a number of factors. Generally full contact aluminium roofs have very good track records in alcohol service even with standard alloys.
Where the contact area is small, such as in non-contact roofs the chance of occurrence of galvanic corrosion is bigger, where this is rarely observed in full contact roofs. The use of more corrosion resistant alloys is important for extension of the expected service life. For full contact roofs expensive treatments such as anodising do not increase the service life significantly, except in services with extremely dry alcohols, such as occur directly after production. In these cases the hot and dry storage conditions trigger “dry corrosion†with high corrosion rates.
For Non-contact roofs the smaller contact area of the pontoons makes it more vulnerable for galvanic corrosion and the effect of impurities in the limited contact area is larger when compared to full contact roofs. Therefore significant degradation of floaters has been observed in rare occasions in Alcohol service. Setting limits for the allowable impurities (such as chlorides), minimum water content and storage temperature of the stored product will make it possible to use aluminium Non-contact roofs for long reliable in service periods without issues. It is, however advised to anodise floaters of non-contact roofs in services where the storage conditions and exact alcohol composition is not readily known.
Taking into consideration the corrosion mechanisms and the influence of storage conditions and selection of roof type and construction materials aluminium internal floating roofs can be used reliably in alcohol service .
1 ROOF CONSTRUCTION
According to API 650-H, the leading standard for atmospheric storage tanks the following types of IFR can be distinguished:
1.1 NON-CONTACT IFR AS PER API 650-H 2.2 E
Metallic internal floating roofs on floats have their deck above the liquid, supported by closed pontoon
compartments for buoyancy. These roof decks are not in full contact with the liquid surface and are typically constructed of aluminium alloys or stainless steel.
- Aluminium or stainless steel floaters in contact with the product
- Rim sections and skirts of penetrations in contact with the product
- Deck skin and roof structure (usually Aluminium) in vapour phase
1.2Â FULL CONTACT IFR AS PER API 650-H 2.2
Metallic sandwich-panel/composite internal floating roofs have metallic or composite material panel modules for buoyancy compartments. Panel modules may include a honeycomb or closed cell foam core; however, cell walls within the panel module are not considered “compartments†for purposes of inspection and design buoyancy requirements (see H.4.1.7 and H.4.2.1).30 These roofs are in full contact with the liquid surface and are typically constructed of aluminium alloys or Purchaser approved composite materials.
- Whole surface in contact with aluminium
2Â CONSTRUCTION MATERIALS
Aluminium has a natural corrosion protection from its oxide layer, but if exposed to aggressive environments it may corrode. Still, if correctly fabricated, constructions of aluminium may be reliable and have long service life.
2.1 CORROSION MECHANISMS
Typically these corrosion mechanisms are considered:
- Oxide destabilisation
- Micro galvanic coupling
- Pitting
- Intergranular corrosion
- Dry corrosion
The comprehensive paper written by Gaute Svenningsen from Norwegian department of Materials Technology, which is added in the addendum gives an excellent insight in these mechanisms.
History shows that 2 and 3 have occurred in Alcohol service.
2. Micro galvanic coupling requires an anode and cathode, that attach the active phases in the aluminium., where locally the composition of the aluminium changes due to the galvanic attack, which changes the corrosion properties significantly.
3. Pitting. Due to destabilization of the oxide layer a pit forms, which is propagated due to lack of re-passivation
- Dry corrosion
If aluminium and magnesium alloys are attacked by alcohol a further type of corrosion is given by literature sources. There, a heavy, localised corrosion with strong gas development with absence of water (<0.1% in ethanol and <0.3% in methanol, depending on temperature) is described. This type of corrosion is called ‘‘dry corrosion’’. Dry corrosion was not relevant in practice for a long time, because the water content of mixed fuels were rarely below 1%. For mixed fuels in Europe a water content below 0.3 vol.% is required (DIN EN 51625). This value must be guaranteed at the petrol pump. Since the water content of alcohol increases during the storage, because of the hygroscopic effect of ethanol and the improved synthesizing process, there are fuels offered with water content below 0.05 vol.% now.
This can lead to dry corrosion. The mechanisms and reasons of this form of corrosion are inadequately explained so far. In a paper by L. Krüger, F. Tuchscheerer, M. Mandel, S. Müller and S. Liebsch, published on November 15th 2011 this phenomenon was investigated. The conclusion is that a water free environment does not allow for the oxide layer to re-form, which introduces a 2 step corrosion mechanism with high corrosion rates and higher risk for sustained corrosion at temperatures above 60 degrees Celsius.
The study has shown that under the present conditions dry corrosion strongly depends on temperature and water content, whereas the content of ethanol and the grain size of the aluminium are of secondary importance. To trigger dry corrosion, the water content must fall below a critical level. In the tests this level was near to 0.2 vol.% water in the ethanol–fuel mixture ad a temperature of 60oC.
2.2 CORROSION RESISTANT ALLOYS
AL 5052 is generally recommended as a suitable aluminium grade for alcohol service, as it has improved corrosion resistance when compared to the standard 3000 series aluminium IFR components. Experience shows that AL 5052 can be used in Alcohol service with expected service life of at least 10 years.
2.3 ANODIZING ALUMINIUM IFR MATERIALS:
By anodising a thick oxide layer will be deposited on the aluminium, reduces the effect of attacks to the layer. This had a positive effect on the service life, but does not take away the cause itself. The corrosion rate in the period that the oxide layer is intact will be very slow. In this respect anodising van be evaluated in the same way as a coating would. It provides protection over a calculated time, after which the attack will be similar to unprotected metal.
3 QUALITY OF THE STORED PRODUCT
As indicated in the corrosion mechanisms water content and temperature of the stored product have a major influence on the suitability of aluminium. Impurities, such as (Ga, Tl, In, Sn, Pb) may become incorporated in the oxide and destabilise it and also the conductivity of the stored product has impact on the roofs’ suitability for service. Generally the higher the conductivity the smaller the chances of high corrosion rates.
Also Chloride content is important. Study shows that a maximum content of 50 ppm Chloride will allow the oxide layer to re-form and passivate the aluminium
4 BACKGROUND ON ALUMINIUM CORROSION, TAKEN FROM A PAPER BY GAUTE SVENNINGSEN
From a purely thermo dynamical point of view aluminium is active. However, in oxygen containing environment (air, water), aluminium is rapidly covered with a dense oxide layer. The oxide layer is essentially inert, and prevents corrosion. The thickness of the layer may vary as a function of temperature, environment and alloy elements. Oxide films formed in air at room temperature are 2-3 nm thick on pure aluminium. Heating to 425°C (or anodising) may give films up to 20 nm [2]. If the oxide film is damaged, e.g. by a scratch, new oxide will immediately form on the bare metal [3]. This way aluminium is given excellent corrosion protection.
The following corrosion mechanisms can occur
4.1 OXIDE DESTABILISATION
The following factors may affect the stability of the aluminium oxide and thereby cause corrosion:
- The oxide is not stable in acidic (pH < 4) or alkaline (pH > 9) environments [1].
- Aggressive ions (chlorides, fluorides) may attack the oxide locally.
- Certain elements (Ga, Tl, In, Sn, Pb) may become incorporated in the oxide and destabilise it [4].
4.2 MICRO GALVANIC COUPLING
Most commercial alloys contain several types of intermetallic phases. Corrosion on aluminium alloys is essentially a micro galvanic process between these phases and the matrix alloy [4]. While the phases often act as local cathodes because of their Fe content, the surrounding aluminium matrix undergoes localized attack. In addition the following phenomena may become important:
- An active phase(s) may corrode preferentially.
- A corroding phase may serve as a sacrificial anode and provide cathodic protection to the surrounding material.
- Due to the electrochemical reactions at the corroding sites and the cathodes, the composition and pH of the electrolyte adjacent to the reaction sites may become different from the bulk electrolyte.
- Active components of the matrix alloy and the intermetallic phases may corrode selectively (dealloying), resulting in changed corrosion properties.
4.3 PITTING CORROSION:
Pitting is a highly localized type of corrosion in the presence of aggressive chloride ions. Pits are initiated at weak sites in the oxide by chloride attack [4,5]. Pits propagate according to the reactions
Al = Al3+ + 3e-Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â (1)
Al3+ + 3H2O = Al(OH)3 + 3H+Â Â Â Â (2)
while hydrogen evolution and oxygen reduction are the important reduction processes at the intermetallic cathodes, as sketched below
2H+ + 2e- = H2Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â (3)
O2 + 2H2O + 4e- = 4OH-Â Â Â (4)
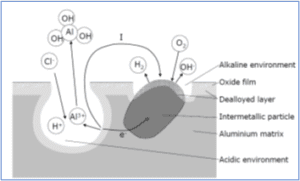
As a pit propagates, the environment inside the pit (anode) changes. According to reaction 2 the pH will decrease. To balance the positive charge produced by reaction 1 and 2, chloride ions will migrate into the pit. The resulting HCl formation inside the pit causes accelerated pit propagation.
The reduction reaction will cause local alkalinisation around cathodic particles. As previously mentioned aluminium oxide is not stable in such environment, and aluminium around the particles will dissolve (alkaline pits). The active aluminium component of the particles will also dissolve selectively, thereby enriching the particle surface with Fe and increasing its cathodic activity. Etching of the aluminium matrix around the particles may detach the particles from the surface, which may re-passivate the alkaline pits. This may also reduce the driving force for the acidic pits causing re-passivation of some in the long run. Figure below shows pitting on an AlMgSi alloy.
4.4Â INTERGRANULAR CORROSION
Intergranular corrosion (IGC) is the selective dissolution of the grain boundary zone, while the bulk grain is not attacked [7]. IGC is also caused by micro galvanic cell action at the grain boundaries. The susceptibility to IGC is known to depend on the alloy composition and thermomechanical processing.
Grain boundaries are sites for precipitation and segregation, which makes them physically and chemically different from the matrix. Precipitation of e.g. noble particles at grain boundaries depletes the adjacent zone of these elements, and the depleted zone becomes electrochemically active. The opposite case is also possible; precipitation of active particles at grain boundaries would make the adjacent zone noble.




